Abstract
Background:
NXT007, emicizumab (Emi)-based engineered therapeutic bispecific antibody which increases tissue factor (TF)-triggered thrombin generation (TG) potential of factor(F) VIII-deficient plasma to non-hemophiliac ranges at around 5-30 μg/mL (Yamaguchi, ASH 2020), is currently in a phase 1/2 clinical study. In the clinical settings under NXT007-prophylaxis, bypassing hemostatic agents (BPAs), such as activated prothrombin complex concentrates (aPCC) and recombinant(r) FVIIa, may be concomitantly administered. Under Emi-prophylaxis, repeated doses of aPCC impose a thrombotic risk. Against the risk, NHF's Medical and Scientific Advisory Council (MASAC) recommends ≤50 U/kg and ≤100 U/kg of aPCC as initial dose and one day dosage, respectively. In case of NXT007-prophylaxis, concomitant-use with BPAs should be also carefully managed and thus basic non-clinical combination data are needed.
Objectives:
To examine in vitro effects of BPAs in the co-presence of NXT007 for providing rough indicators for determining their safe doses.
Methods:
First, TF-triggered TG assay was performed using commercial FVIII-deficient plasma. rFVIII, rFVIIa or aPCC was spiked in the co-presence of NXT007 or Emi (0.1-50 μg/mL). Second, the TG assay and whole blood clot viscosity test (ROTEM) using Ca 2+-trigger was performed using healthy volunteer's blood incubated with anti-FVIII antibodies (HA model), where aPCC or rFVIIa was spiked in the co-presence of NXT007. Third, ROTEM was performed as above using whole blood from persons with hemophilia A (PwHA) under Emi- or FVIII-prophylaxis.
Results:
Peak height of TF-triggered TG assay using FVIII-deficient plasma was increased by spiking each of the agents (rFVIII, rFVIIa or aPCC) in the co-presence of NXT007 (0.1-50 μg/mL). Peak height increase by rFVIII under NXT007 was roughly additive. Peak height increase by each BPA under NXT007 was synergistic. Synergistic effect by aPCC was more intensive than that by rFVIIa. The combination effect of 0.1 U/mL aPCC and 0.1-50 μg/mL NXT007 on peak height did not exceed that of 0.5 U/mL aPCC and 50 μg/mL Emi. Peak height at 2 μg/mL NXT007 alone was comparable to that at 50 μg/mL Emi alone. When adding BPAs to these two settings, similar synergistic effects were observed. It suggested that NXT007's combination actions with BPAs were qualitatively similar to Emi's.
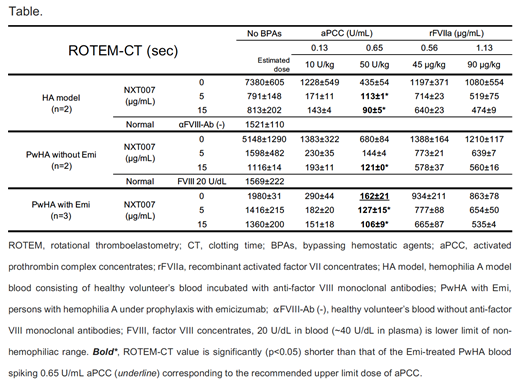
In the TG assay using the HA model plasma (n=2), the combined effect of aPCC and NXT007 was similarly confirmed. In ROTEM using the same HA model whole blood (n=2), clotting time (ROTEM-CT, 7380±605 sec) was shortened by spiking 5 μg/mL NXT007 (791±148 sec) to yield normal levels (1521±110 sec). Spiked aPCC (0.13, 0.65 U/mL in whole blood, equivalent to ~10, ~50 U/kg infusion) shortened ROTEM-CT (1228±549, 435±54 sec) and further shortened it in the co-presence of 5 or 15 μg/mL NXT007 (171±11, 113±1* or 143±4, 90±5* sec [* significantly ( p<0.05) shorter than that of the Emi-treated PwHA blood spiking 0.65 U/mL aPCC, 162±21 sec)].
In PwHA without Emi-prophylaxis (n=2), ROTEM-CT (5148±1290 sec) was shortened by spiking 5 or 15 μg/mL NXT007 (1598±482 or 1116±14 sec) to non-hemophiliac levels (20 IU/dL rFVIII, 1569±222 sec). Spiked aPCC (0.13, 0.65 U/mL) shortened ROTEM-CT (1383±322, 680±84 sec) and further shortened it in the co-presence of 5 or 15 μg/mL NXT007 (230±35, 144±4 or 193±11, 121±0* sec). Spiked rFVIIa also shortened ROTEM-CT in the co-presence of NXT007, but the intensity was less than spiked aPCC.
ROTEM using blood from PwHA under Emi-prophylaxis (n=3) were also performed and demonstrated that the effects by co-spiking BPAs and NXT007 were roughly consistent with those using PwHA blood without Emi-prophylaxis. These ROTEM data indicated that the combined effect of 0.13 U/mL aPCC and 5-15 μg/mL NXT007 was less intensive than that of 0.65 U/mL aPCC spiked to the Emi-treated PwHA blood, while that of 0.65 U/mL aPCC and 5-15 μg/mL NXT007 had more intensive effect (Table).
Conclusion:
In considering concomitant use of BPA under NXT007-prophylaxis, dose of aPCC should be more carefully determined than that of rFVIIa. In this non-clinical study, the combined effect of ~0.13 U/mL aPCC (equivalent to ~10 U/kg infusion) and ~15 μg/mL NXT007 did not exceed that of 0.65 U/mL aPCC (50 U/kg infusion) under Emi-prophylaxis situation corresponding to the upper limit of initial concomitant dose recommended by MASAC, which might be rough indicators in future clinical settings.
Ogiwara: Chugai Pharmaceutical Co., Ltd.: Consultancy, Research Funding. Furukawa: Chugai Pharmaceutical Co., Ltd.: Research Funding. Sasai: Chugai Pharmaceutical Co., Ltd.: Research Funding. Inaba: Chugai Pharmaceutical Co., Ltd.: Current Employment. Kitazawa: Chugai Pharmaceutical Co., Ltd.: Current Employment, Current equity holder in publicly-traded company. Nogami: Chugai Pharmaceutical Co., Ltd.: Consultancy, Research Funding.